Have you ever wondered why your ice cream melts on a hot summer day or why water turns into steam when boiled? Join us on a whimsical journey through the wacky world of matter as we explore solids, liquids, gases, and maybe even a few states of matter you didn’t know existed. So grab your lab coat and safety goggles, because things are about to get (chemically) wild!
Overview of Matter Phases
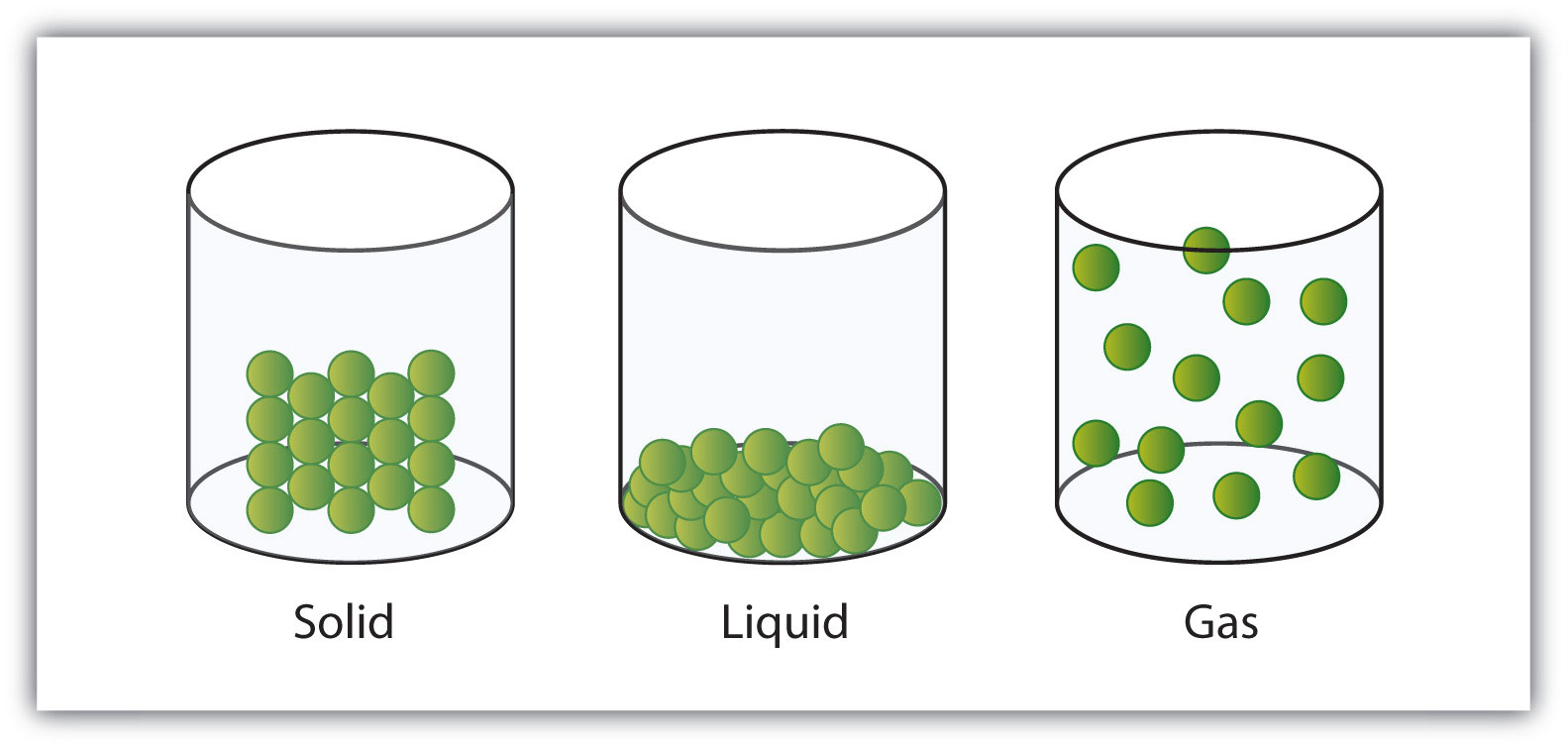
Ever looked at a solid, a liquid, and a gas and wondered what they all have in common? That’s right, they’re all different phases of matter! Below, we’ll take you through the wacky world of matter phases.
First up, we have solids. Picture your favorite superhero – solid as a rock and tough as nails. Solids are just like that, with particles packed tightly together, giving them a definite shape and volume. They’re not great at parties, though, since they’re quite stiff and boring.
Next, we have liquids. Imagine a liquid as your fun-loving best friend - always flowing and taking the shape of its container. With particles that move around more freely than in solids, liquids have a definite volume but no definite shape. They’re the life of the party, always ready to mix it up!
Finally, we have gases. Gases are like that friend who’s always disappearing on you – they’ll take up as much space as they can get their hands on! With particles flying around like crazy, gases have neither a definite shape nor volume. They’re the wild cards of the matter world, always ready for a spontaneous adventure.

Solid State: Structure and Properties
When it comes to solid state materials, the structure and properties are like the peanut butter and jelly of the science world - they just go together perfectly!
Have you ever stopped to think about how solid state materials are kind of like a group of really well-behaved atoms, all snuggled up together in a nice, orderly fashion? It’s like they’re having a little atomic slumber party, all keeping each other in line so they don’t cause any trouble.
And let’s not forget about the properties of these solid state materials – they’re like the personality traits that make each material unique. From conductivity to magnetism, these properties give solid state materials their own special charm.
So, next time you come across a solid state material, take a moment to appreciate the structure and properties that make it so special. Who knew that science could be so much fun!

Liquid State: Characteristics and Behavior
Imagine a world where everything flows freely like a never-ending river. That’s the liquid state for you! Liquids are like the cool kids of the molecular world – always on the move, never conforming to a rigid structure. But what exactly makes them so special? Let’s dive into the characteristics and behavior of liquids and uncover the secrets of their watery ways.
First and foremost, liquids have a unique way of taking the shape of their container. It’s like they’re chameleons, blending in effortlessly wherever they go. This fluidity allows them to adapt to any situation – whether they’re sloshing around in a glass of water or swirling in a vortex. Talk about versatility!
Another fascinating characteristic of liquids is their ability to flow and pour. Unlike solids that stay put like stubborn rocks, liquids are constantly in motion, gliding and meandering with graceful ease. They’re the smooth operators of the molecular world, effortlessly slipping and sliding wherever they please.
But beware – liquids can also be a tad rebellious at times. Just when you think you have them figured out, they might surprise you with their capricious behavior. One moment they’re calm and collected, and the next they’re bubbling and boiling like a mini volcano. It’s like trying to tame a wild river – you never know what they’ll do next!
Gas State: Properties and Kinetic Theory
Gas particles are like the mysterious free spirits of the material world, bouncing around and never staying in one place for too long. They may not have a solid form, but they sure do have some interesting properties that make them stand out from the rest of the states of matter.
One key property of gases is their ability to expand to fill the container they are placed in. It’s almost like they have a case of FOMO (Fear Of Missing Out) and they need to make sure they don’t miss a single corner of the space. This ability makes them perfect for filling up balloons at parties or making sure your car tires are adequately inflated.
According to the Kinetic Theory, gas particles are constantly in motion, bumping into each other and the walls of their container. It’s like they’re playing a never-ending game of tag, except instead of tagging each other, they’re sharing their energy through collisions. This constant movement is what gives gases their high pressure and low density – they just can’t sit still!
So next time you see a balloon floating in the air or feel the refreshing breeze on a hot summer day, remember that it’s all thanks to the unique properties and kinetic energy of gas particles. They may be invisible, but they sure do know how to make their presence known!
Plasma State: Understanding the Fourth State of Matter
Plasma, the elusive fourth state of matter, is like that one friend who always shows up to the party fashionably late but steals the show with their electrifying presence. This supercharged state is created when gas molecules are heated to extreme temperatures, causing them to ionize and become electrically conductive.
Imagine a chaotic dance floor at a rave, where positively charged ions and negatively charged electrons are bumping and grinding in a fiery frenzy. It’s like a cosmic tango between particles, where boundaries are blurred and anything goes. This wild behavior gives plasma its unique properties, such as the ability to respond to electromagnetic fields and emit light.
So, what can we learn from this electrifying state of matter? Well, for starters, plasma plays a crucial role in our universe, from the scorching sun to neon signs lighting up city streets. It’s like the rockstar of the cosmos, bringing light and energy to the party. Plus, scientists are harnessing the power of plasma for technologies like plasma TVs and fusion reactors, proving that sometimes the best party tricks come from the most unexpected guests.
Bose-Einstein Condensate: The Coldest State in the Universe
What is Bose-Einstein Condensate?
Imagine a place where atoms are so chill, they’re practically frozen solid. That’s right, we’re talking about the coldest state in the universe – the Bose-Einstein Condensate. At temperatures just a hair above absolute zero, atoms in this state move as if they’re in slow motion, giving new meaning to the phrase “cool as a cucumber.”
How is it created?
Creating a Bose-Einstein Condensate is no easy feat. Scientists must first trap a bunch of ultra-cold atoms in a magnetic or optical trap. Then, they slowly lower the temperature until the atoms reach a point where they can no longer maintain their individuality, merging together to form a single quantum entity. It’s like the ultimate chill party where everyone becomes best buds.
What can we learn from it?
The Bose-Einstein Condensate may be cold, but it’s hot stuff in the world of physics. Scientists study this state of matter to learn more about quantum mechanics, superfluidity, and even the nature of time itself. Who knew that something so chill could hold the key to unlocking the mysteries of the universe?
Exploring Exotic Phases of Matter: Superfluids and Supersolids
Remember those boring old states of matter like solid, liquid, gas? Well, get ready to have your mind blown because we’re diving deep into the world of superfluids and supersolids – the cool kids of the material science world!
First up, let’s talk about superfluids. Imagine a substance that can flow without any friction whatsoever. Yep, that’s right, no resistance at all. It’s like the VIP section of the material world, where particles move in perfect harmony, never bumping into each other like clumsy dancers at a middle school prom.
Now, onto supersolids. It’s like a solid and a superfluid had a baby and created this mind-bending state of matter. Picture a substance that is solid and can still flow like a liquid. It’s like trying to catch a ghost - elusive, mysterious, and totally fascinating.
So grab your lab coat and safety goggles, because we’re about to embark on a wild ride through the strange and exotic realms of superfluids and supersolids. Who knew science could be this cool? Stay tuned for more mind-blowing discoveries!
FAQs
What’s the deal with solids, liquids, and gases?
Well, my dear reader, imagine if you will a lively dance party. Solids are like those wallflowers who just don’t want to leave their spot on the dance floor. Liquids, on the other hand, are the ones grooving and flowing around in a more relaxed manner. And gases? They’re the wild ones, bouncing around with no care in the world!
Are there any other phases of matter besides solids, liquids, and gases?
Absolutely! There are a couple more cool kids on the block. Ever heard of plasma? It’s like the rebellious teenager of the group, super energetic and always causing a commotion. And then there’s Bose-Einstein condensate, the mysterious and chill one who likes to hang out at near absolute zero temperatures. So yeah, it’s a pretty diverse crew!
Can matter change from one phase to another?
Oh, for sure! It’s like a makeover montage in a teen movie. Solids can melt into liquids, liquids can evaporate into gases, and gases can condense back into liquids. And if you’re feeling fancy, you can even zap some matter into a plasma state with a bit of heat!
Why is it important to study the phases of matter?
Well, dear reader, understanding the phases of matter is like having a superpower in your back pocket. It helps us make sense of the world around us, from cooking up a storm in the kitchen to launching rockets into space. Plus, it’s just plain fascinating to unravel the mysteries of the universe!
—
Thanks for Diving into the States of Matter Madness!
Congratulations, you’ve now become a certified expert in the wild world of solids, liquids, gases, and even plasma! We hope you had a blast exploring the different phases of matter and learning all about their quirky behaviors.
Remember, whether you’re chilling out as a solid, going with the flow as a liquid, or bouncing around like a gas, there’s always more to discover in the endlessly fascinating realm of physics. So keep on experimenting, keep on observing, and most importantly, keep on having fun with science!
Until next time, stay curious and keep your state of matter in check!