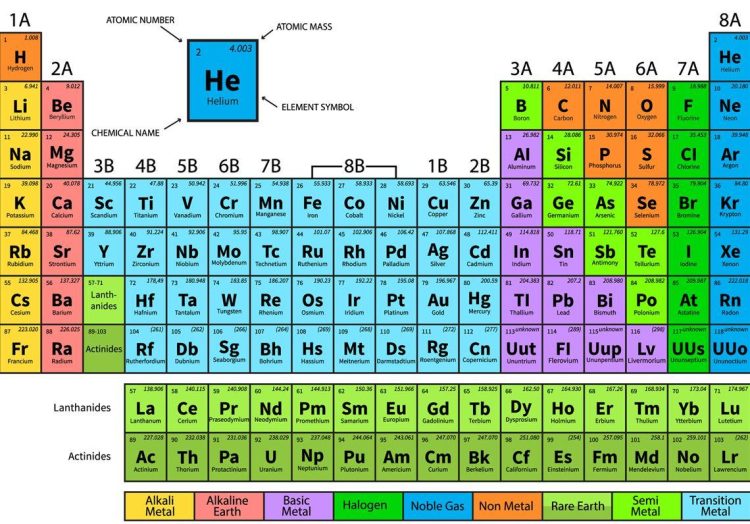
Buckle up, fellow science enthusiasts, because we are about to embark on a cosmic journey to unlock the secrets of the universe’s very own recipe book - the Periodic Table! Like a quirky alchemist’s guide to the building blocks of the cosmos, this colorful chart holds the key to decoding the elemental blueprint of all things, from the tiniest atom to the grandest star. So grab your lab coats and safety goggles, because we’re about to dive deep into the wacky world of chemistry and discover just how weird and wonderful our universe truly is!
The Origins and Evolution of the Periodic Table
The periodic table is a fascinating creation that has evolved over centuries to become the organized masterpiece we know today. Imagine a chaotic mess of elements swirling around in confusion, desperately trying to find their place in the universe. It’s like a cosmic game of musical chairs, but instead of chairs, it’s empty slots waiting to be filled by quirky elements.
From the early attempts to categorize elements based on their properties to Mendeleev’s stroke of genius in creating the first recognizable periodic table, this iconic chart has seen it all. Elements like hydrogen and helium were like the popular kids in high school, always hanging out together at the top of the table, while the noble gases were the loners, preferring to keep to themselves on the far right.
As scientists discovered more and more elements, the periodic table grew and expanded, like a never-ending jigsaw puzzle. New rows and columns were added, filling in the gaps and creating a colorful mosaic of elements. Each element had its own story to tell, from the volatile alkali metals to the mysterious lanthanides and actinides.
Today, the periodic table stands as a testament to human ingenuity and curiosity. It’s a constant reminder that the universe is a vast and diverse place, full of surprises and wonders waiting to be uncovered. So the next time you glance at the periodic table, remember that behind each element lies a rich history and a tale of evolution that spans centuries.
The Building Blocks of Matter: Understanding Elements
When it comes to understanding elements, it all boils down to the building blocks of matter. These tiny particles make up everything around us, from the air we breathe to the ground we walk on. Let’s dive into the fascinating world of elements and uncover their secrets!
First up, we have **atoms**. These little guys are the basic units of matter, kind of like the LEGO pieces of the universe. They’re made up of even tinier particles called protons, neutrons, and electrons. It’s like a teeny tiny family all living together in harmony.
Next, we have **elements**, which are made up of one specific type of atom. Think of them as the unique characters in a play – each element has its own special qualities and quirks. They’re like the spice of life, adding flavor to the world around us.
Now, let’s talk about the **periodic table**. This handy-dandy chart organizes all the elements based on their properties and atomic structure. It’s like a giant cheat sheet for the building blocks of the universe. So next time you’re feeling lost in the world of elements, just remember – it’s all just a puzzle waiting to be solved!

The Periodic Table: A Systematic Arrangement of Elements
Ever wondered how scientists manage to keep track of all the different elements in the universe? Well, let me introduce you to the periodic table – a magical chart that organizes all the elements in a systematic and orderly manner.
Picture this: a grand banquet hall where all the elements have been assigned a seat at the table according to their atomic number and properties. It’s like a sophisticated seating plan for a fancy dinner party, except with atoms instead of fancy guests.
Each element has its own unique personality – from the rebellious and explosive nature of hydrogen to the stable and reliable nature of iron. It’s like a never-ending soap opera with elements forming bonds, breaking apart, and causing chemical reactions left and right.
So next time you’re feeling overwhelmed by the endless possibilities of the periodic table, just remember that it’s simply a way to bring order to the chaotic world of atoms. Embrace the organized chaos and marvel at the beauty of this systematic arrangement of elements!
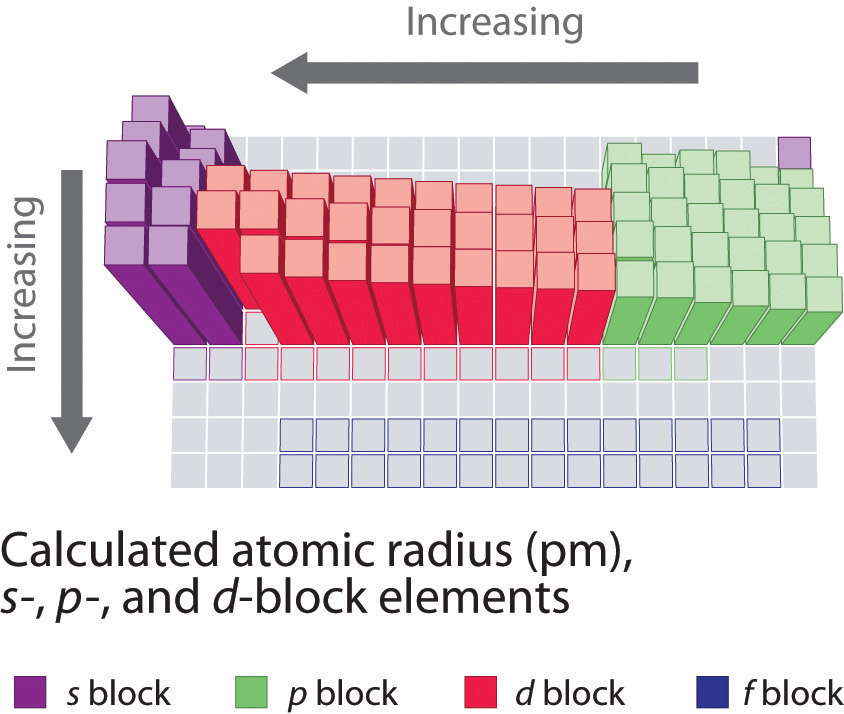 Patterns and Trends in the Periodic Table”>
Patterns and Trends in the Periodic Table”>
Unraveling the Patterns and Trends in the Periodic Table
Have you ever stared at the periodic table and wondered what all those numbers and symbols were trying to tell you? Well, fear not, dear reader, for we are about to embark on a journey to unravel the mysterious patterns and trends that lurk within the confines of the table.
First off, let’s talk about the noble gases. These guys think they’re so cool with their full outer electron shells, but really they’re just a bunch of snobs who refuse to play with the other elements. You’ll find them hanging out all smug-like in Group 18, just basking in their inert glory.
Then there’s the alkali metals, those wild and crazy folks in Group 1 who are always itching to give away their outer electron like it’s a hot potato. They’re like the party animals of the periodic table, causing explosions and setting things on fire just for kicks.
And let’s not forget about the halogens, the drama queens of Group 17 who can’t seem to make up their minds whether they want to gain or lose electrons. They’re like that friend who’s always starting arguments and causing chaos wherever they go.
 Properties“>
Properties“>
The Role of Electrons in Determining Element Properties
In the wild world of chemistry, electrons are like the cool kids at the party - they determine everything about an element’s personality. These tiny particles whizz around the nucleus, causing all sorts of mischief and influencing how an element behaves. It’s like they have their own secret code language that only other electrons can understand.
Think of electrons as the ultimate matchmaker, pairing up with other elements to create new compounds. They’re like the ultimate wingmen, always looking out for their element buddies and helping them bond with others. Without electrons, elements would be totally lost in the dating scene of the periodic table.
When it comes to determining an element’s properties, electrons are the ultimate influencers. They dictate everything from an element’s reactivity to its color, smell, and taste (just kidding, please don’t taste elements). It’s like they have the power to turn an element from a shy wallflower into the life of the party, just by moving around and sharing their energy.
So next time you’re geeking out over the periodic table, remember to give a little shoutout to those electrons – they may be small, but they sure know how to throw a chemical party!
The Periodic Table as a Tool for Predicting Chemical Behavior
Ever wondered how chemists are able to predict the behavior of elements in a reaction? Well, look no further than the trusty periodic table! This handy tool organizes all the elements in a way that allows us to make educated guesses about how they will interact with each other.
One of the most useful things about the periodic table is its arrangement of elements based on their atomic number and electron configuration. This organization helps us understand trends in properties such as atomic size, electronegativity, and reactivity.
For example, elements in the same group (vertical column) tend to have similar chemical behavior because they have the same number of valence electrons. This means they are likely to form similar types of compounds and react in similar ways. So, if you know how one element in a group behaves, you can make an educated guess about how others in the same group will behave as well.
So next time you’re trying to predict the outcome of a chemical reaction, just remember to consult your trusty friend, the periodic table. It may not be able to brew you a cup of coffee, but it sure can help you understand why adding sugar to your morning brew makes it taste so sweet!
The Modern Application of the Periodic Table in Scientific Research
is truly fascinating. Scientists are utilizing this iconic chart in ways never thought possible, pushing the boundaries of what we know about the elements and their properties.
One of the most intriguing uses of the Periodic Table is in the field of nuclear chemistry, where researchers are exploring the stability and reactivity of various isotopes. By referencing the table, scientists can predict how different elements will behave in nuclear reactions, helping to further our understanding of nuclear energy and radiation.
Another cutting-edge application of the Periodic Table is in materials science, where scientists are developing new materials with unique properties and applications. By understanding the periodic trends of the elements, researchers can design materials with specific characteristics, such as superconductivity or high strength-to-weight ratios.
Overall, the Periodic Table continues to play a vital role in scientific research, guiding scientists in their quest to unlock the mysteries of the elements and advance our understanding of the world around us. Who knew a chart created in the 19th century could still be so relevant and useful in the 21st century!
FAQs
Why is the periodic table considered the “blueprint” of the universe?
Well, imagine the universe as a giant LEGO set. The periodic table is like the instruction manual that tells you how to put all the pieces together to create everything around us. It’s the ultimate cheat sheet for understanding the building blocks of the cosmos.
How did scientists come up with the periodic table?
Back in the day, scientists were like the Sherlock Holmes of chemistry, searching for clues to figure out the patterns and relationships between different elements. Eventually, Mendeleev cracked the code and organized the elements into the table we know and love today. It was like solving a cosmic Sudoku puzzle!
What can the periodic table tell us about the properties of elements?
Oh, it’s like having a crystal ball that shows you the superpowers of each element. Whether it’s being a shiny metal, a stinky gas, or a radioactive weirdo, the periodic table reveals all the quirks and characteristics of the elements. It’s like flipping through a cosmic Tinder for chemical elements!
Why is it important to study the periodic table?
Think of the periodic table as the Rosetta Stone of chemistry. By understanding its hidden messages and patterns, scientists can unlock the secrets of the universe, create new materials, and even blow stuff up (safely, of course). It’s like having a superpower that lets you manipulate the very fabric of reality!
—
And that’s a wrap!
Congratulations on taking your first step towards becoming a chemistry wizard! We hope this article helped you unlock the mysteries of the periodic table and inspired you to delve deeper into the world of elements. Remember, the periodic table isn’t just a chart of boring numbers and symbols – it’s the ultimate cheat sheet that decodes the universe’s elemental blueprint! So go forth, young scientist, and may your experiments be full of exciting discoveries and minimal explosions!