Welcome fellow water enthusiasts! Have you ever stopped to appreciate the miraculous properties of H2O? From sticking together like a clingy ex (cohesion) to getting along famously with other substances (adhesion), water is truly the social butterfly of the chemical world. And let’s not forget about surface tension – the invisible force that turns water into a tightrope walker. So grab your lab coat and magnifying glass, because we’re diving deep into the mesmerizing world of water properties. Let’s make a splash!
Understanding Cohesion in Water Molecules
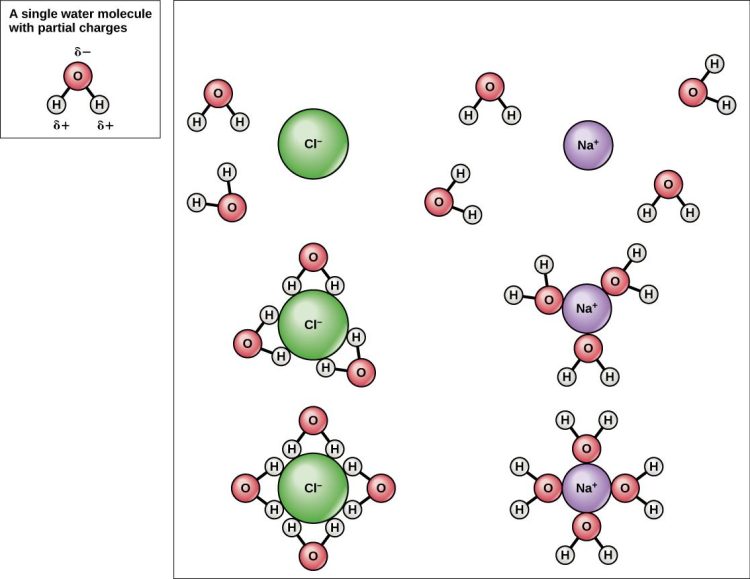
Water molecules are like that clingy friend who just can’t seem to let go. They are so obsessed with each other that they stick together like glue, forming strong bonds known as cohesion.
But what exactly is this mystical force that keeps water molecules so tightly knit? Well, it all comes down to the magic of hydrogen bonding. You see, water molecules are made up of two hydrogen atoms and one oxygen atom. These little guys are like the ultimate power trio, with hydrogen playing the role of the loyal sidekick.
When water molecules come together, the hydrogen atoms from one molecule bond with the oxygen atom of another, creating a chain reaction of love and affection. This bonding creates a strong attraction between the molecules, keeping them close and cozy.
So next time you take a sip of water, just remember that those molecules are holding hands tighter than a couple on their first date. And that, my friends, is the beauty of cohesion in water molecules.

The Role of Adhesion in Water’s Behavior
Have you ever stopped to think about how water behaves in such a clingy way? Well, let me tell you, adhesion plays a huge role in water’s behavior. It’s like water just can’t seem to let go of anything it comes into contact with!
Adhesion is like that friend who always tags along when you’re just trying to have a relaxing time by yourself. Water molecules are constantly reaching out and forming bonds with other substances, whether it’s the sides of a glass, a leaf in a pond, or even the walls of your own cells. It’s like they just can’t help themselves!
And let’s not forget about cohesion, water’s other clingy behavior. Cohesion is like when your friends just won’t stop talking in a group chat, and water molecules are the ones who never want to break apart from each other. They stick together like glue, creating those beautiful water droplets we see on windows or leaves after a rainstorm.
So next time you see water droplets forming on a surface or notice how water curves up against the edges of a glass, remember that it’s all thanks to adhesion. It’s like water just can’t bear to let go, always holding on for dear life!
Examining the Concept of Surface Tension
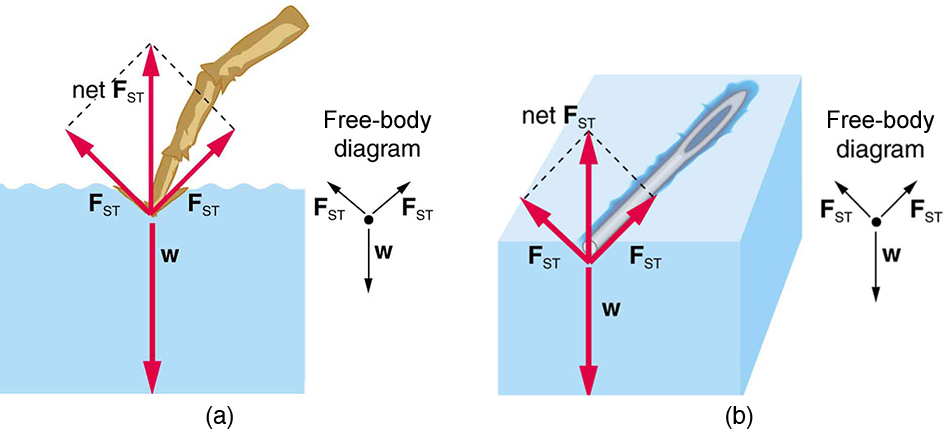
Surface tension, what a strange and mysterious force! It’s like the superhero of liquids, holding everything together and keeping them from spilling all over the place. But how exactly does it work? Let’s dive into the fascinating world of surface tension and see if we can uncover its secrets!
Imagine a group of water molecules hanging out at the surface of a glass of water, having a little party. They’re all connected to each other through their hydrogen bonds, forming a tight-knit community. This strong bond between molecules is what gives water its high surface tension, making it act like a thin, invisible film that can support small objects like paper clips or even bugs. It’s like water is saying, “I got you, buddy!”
But surface tension isn’t just about water showing off its superhero powers. Other liquids like mercury and soap also have surface tension, although they might not be as flashy about it. It’s like they’re the shy sidekicks of the superhero world, quietly doing their job without drawing too much attention to themselves. Hey, even superheroes need a break sometimes!
So, next time you see a droplet of water clinging to a leaf or a bug skittering across the surface of a pond, just remember that it’s all thanks to the magical powers of surface tension. Who knew that something as simple as water could have such a cool trick up its sleeve? Surface tension, you’re one impressive force to be reckoned with!

Impacts of Cohesion and Adhesion on Plant Life
Imagine a world where plants have no friends. They would wilt away, never able to stand tall and proud. This is where cohesion and adhesion come into play, working together like a dream team to keep plants thriving and flourishing.
One of the key is their ability to transport water and nutrients throughout the entire plant system. Cohesion helps water molecules stick together, creating a continuous flow from the roots all the way to the leaves. Adhesion, on the other hand, allows water to stick to the walls of the plant’s vascular system, preventing it from evaporating into thin air. Talk about teamwork!
Not only do cohesion and adhesion help with water transport, but they also play a crucial role in helping plants defy gravity. Thanks to these two forces, plants are able to stand upright, reaching for the sun and soaking in its glorious rays. Without cohesion and adhesion, plants would be limp, sad creatures, unable to support themselves and thrive in their natural environment.
So next time you see a beautiful, vibrant plant swaying in the breeze, take a moment to appreciate the hard work of cohesion and adhesion. They may not be the flashiest players on the team, but they sure do know how to keep plant life in tip-top shape!

Practical Applications of Surface Tension in Daily Life
Imagine a world without the wonders of surface tension – we’d be living in chaos! But fear not, my friends, for surface tension is always right by our side, helping us out in our daily lives in the most practical of ways.
Have you ever noticed how water droplets bead up on a freshly waxed car? That’s surface tension at work, my friends! It’s like mother nature’s way of giving your car a nice little massage. So next time you’re admiring your shiny ride, remember to give a nod of thanks to surface tension.
Ever wonder how ants can walk on water without sinking? It’s all thanks to surface tension! Those little critters are like tiny superheroes, defying gravity with every step they take. I mean, if I could walk on water, I’d probably never leave the pool.
And let’s not forget about everyone’s favorite party trick – the famous floating paperclip! Thanks to surface tension, that little guy can chill on the water’s surface like it’s no big deal. It’s like a magic trick that never gets old.
FAQs
Why do water molecules stick together?
Well, it seems water molecules are just like best friends at a sleepover – they can’t bear to be apart! Thanks to cohesion, the positive and negative charges within the molecules attract each other, creating a bond that is stronger than your grandma’s famous glue.
What’s the deal with adhesion?
Adhesion is like the water molecule’s side hustle - it’s the attraction between water molecules and other substances. Think of it as the water molecules trying to make friends with whatever surface they come into contact with. It’s like they’re constantly looking for new BFFs.
Why does water form droplets on a leaf rather than spreading out?
Ah, the magic of surface tension! Just imagine water as a group of shy introverts at a party – they stick together on the surface, refusing to break out of their comfort zone. This creates droplets instead of spreading out, so next time you see a water droplet on a leaf, remember it’s just a group of socially awkward water molecules.
How does surface tension help insects walk on water?
Oh, those crafty little insects and their water-walking skills! Surface tension acts like a trampoline for them - it’s a thin layer on the surface that can support their weight without breaking. It’s like the surface of the water is saying, “Come on in, the water’s fine…for walking, that is!”
—
Dive into the Wondrous World of Water
As we reach the end of our exploration into the fascinating properties of water, we hope you’ve come to appreciate just how amazing this simple molecule truly is. From its ability to stick to itself like glue (thanks cohesion!) to its knack for sticking to other surfaces (ah, adhesion!), and let’s not forget about that surface tension that keeps bugs from sinking, water never ceases to amaze. So next time you take a sip of water or watch a raindrop cling to a leaf, remember the incredible forces at work in every drop. Stay curious and keep exploring the wonders of our watery world!