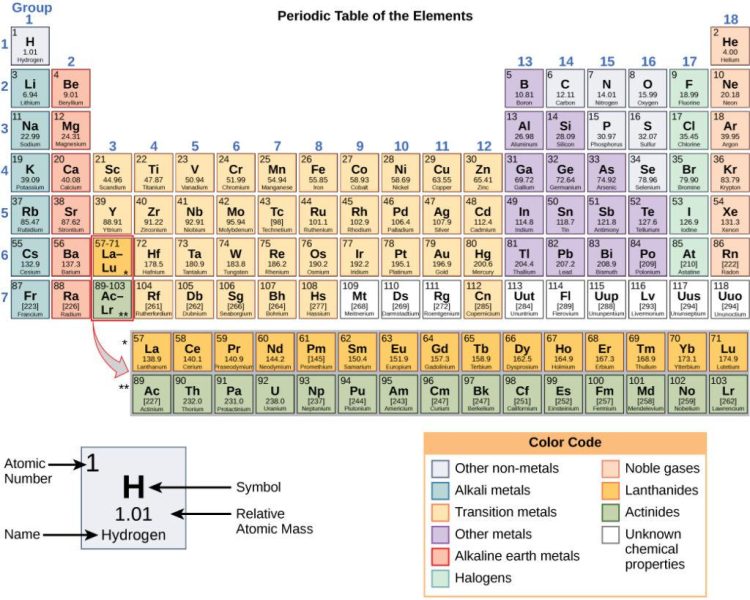
Buckle up, fellow science enthusiasts, because we’re about to embark on a wild ride through the wacky world of chemical elements! From lithium to uranium, we’ll be diving deep into the atomic structures that make up our universe, all while trying not to get too lost in the periodic table maze. So grab your safety goggles and get ready for some element-ary fun!
Overview of the Periodic Table
So you thought the Periodic Table was just a boring chart with a bunch of elements? Think again! Let me give you a mind-blowing overview of this seemingly simple table.
First off, did you know that the Periodic Table is like a giant roadmap for the building blocks of the universe? It’s like Google Maps for atoms, telling us where to find all the different elements and how they relate to each other.
But wait, there’s more! The Periodic Table is not just a random jumble of elements – oh no, it’s organized in a super cool way. Elements with similar properties are grouped together in columns, called groups or families. And there’s even a special group called the Noble Gases, where the elements are too cool to react with anyone else. Talk about being snobbish!
And let’s not forget about the periodic trends – no, not the latest fashion fads, but the patterns that certain properties of elements follow as you move across a row or down a column. It’s like a secret code that gives us insight into the behavior of atoms. Who knew chemistry could be so mysterious?
.jpg)
Description of Atomic Number and Atomic Mass
In the wacky world of atoms, each element has its own unique identity defined by two key characteristics – the atomic number and atomic mass. Let’s break it down in an atomic-sized nutshell, shall we?
**Atomic Number:**
This little number is like the element’s driver’s license – it tells you how many protons are packed into the nucleus. Think of it as the VIP pass that lets the element into the exclusive proton club. The atomic number is the element’s way of saying, “Hey, I’m helium and I’ve got 2 protons - don’t mess with me!”
**Atomic Mass:**
Now, let’s talk about the element’s weight – the atomic mass. This value is like a high-stakes guessing game where you have to balance the protons, neutrons, and electrons. It’s the element’s way of flexing its atomic muscles and showing off its combined proton and neutron mass. So when carbon struts its stuff with an atomic mass of 12.01, you better believe it’s got some serious mass appeal.
So there you have it, folks – the quirky world of atomic number and atomic mass. It’s a wild ride through the periodic table, but once you grasp these concepts, you’ll be the life of every chemistry party. So go forth, intrepid atomic explorers, and remember – protons are friends, not foes!
Explanation of Electron Configuration
So you know those little guys that like to hang out around the nucleus of an atom? Yeah, electrons. They’re kind of like the party animals of the atomic world. But just like us humans, they like to have their own space and organize themselves in a very particular way. This is where electron configuration comes into play.
Picture this: the nucleus of an atom is the dance floor and the electrons are the dancers. Each electron has its own unique “dance move” or orbital that it likes to groove in. The electron configuration is basically just a fancy way of describing how these electrons are arranged around the nucleus.
Now, there’s a method to this madness. Electrons like to follow certain rules when it comes to their configurations. For example, they prefer to fill up orbitals in a way that minimizes their energy. Think of it like choosing the comfiest seat at the party – no one wants to be stuck in an uncomfortable corner.
So, the next time you’re trying to wrap your head around electron configuration, just remember that it’s all about the electrons finding their perfect dance partner (or orbital) and making sure everyone is having a good time around the nucleus. It’s like the atomic version of a well-organized dance party!

Discussion of Valence Electrons
Valence electrons are the cool kids of the atom party. They’re the ones hanging out on the outer energy levels, causing trouble and making chemical reactions happen. Without them, atoms would be like a party with no DJs – boring and uneventful.
Think of valence electrons like the popular kids in high school. They’re the ones with all the friends (other electrons) and the influence to make things happen. They’re the ones that determine an atom’s chemical behavior, much like how the captain of the football team dictates the school’s social scene.
When it comes to bonding, valence electrons are like the matchmakers of the atom world. They’re the ones responsible for bringing different atoms together, whether it’s through ionic bonds, covalent bonds, or just good old-fashioned sharing. Without them, atoms would be lonely and unfulfilled.
So next time you’re feeling down about your own electron configuration, just remember – valence electrons are the life of the atom party. They’re the ones making things happen, causing reactions, and keeping the chemical world spinning. Without them, we’d all be stuck in boring, stable atoms with no chance for excitement. Long live the valence electrons!
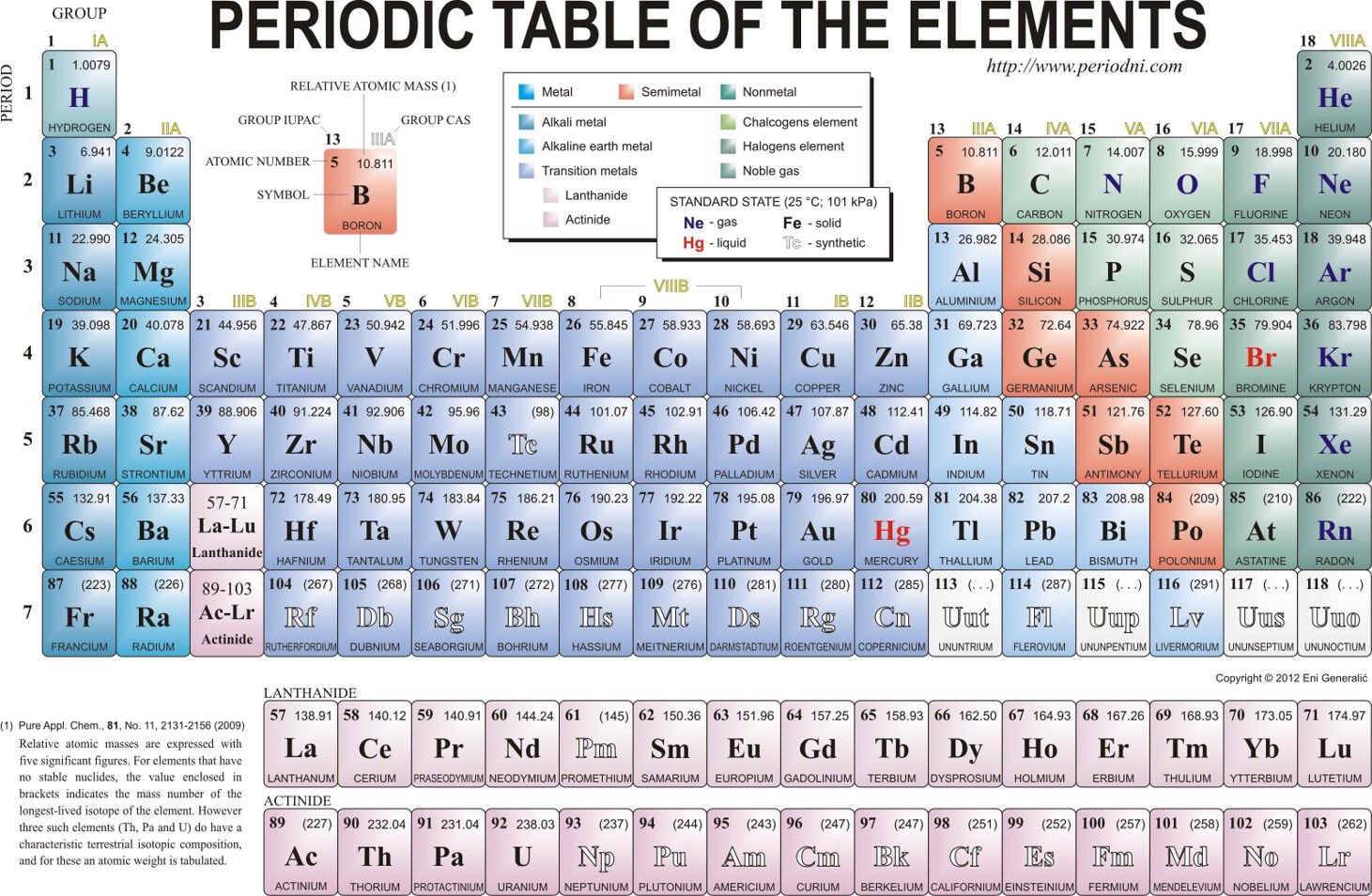
Overview of the Different Element Groups
Alkali Metals:
- These are the rebels of the periodic table, always ready to cause a reaction.
- Watch out for them, they can explode on contact with water!
Transition Metals:
- These elements are like the chameleons of the periodic table, always changing their oxidation states.
- They are essential for many industrial processes and technologies.
Halogens:
- Watch out for these elements, they can be quite toxic.
- Don’t mess with them, they are always ready to form a strong bond.
Noble Gases:
- These elements are the aristocrats of the periodic table, always staying noble and unreactive.
- They are used in lighting, like neon signs, and even in balloons for parties!
Explanation of Isotopes and their Importance in Understanding Elements
Let’s dive into the atomic world and explore the fascinating realm of isotopes! Isotopes are like the quirky siblings of elements – they have the same number of protons (aka the element’s identity), but they differ in the number of neutrons. It’s like having a family of carbon atoms, where some are super buff with extra neutrons while others are more chill with fewer neutrons.
But why should we care about these isotopes? Well, they can tell us a lot about an element’s behavior. Think of isotopes as the element’s different personalities – some are stable and dependable, while others are unstable and prone to radioactive decay. By studying isotopes, scientists can track chemical reactions, understand how elements form, and even date ancient artifacts. It’s like having a spy network of isotopes that reveal the secret lives of elements!
So, next time you see an element, remember that it’s not just a simple atom – it’s a whole family of isotopes waiting to spill the atomic beans. Embrace the diversity of isotopes and marvel at their importance in decoding the mysteries of elements. Who knew that something as tiny as a neutron could have such a big impact on our understanding of the atomic world? It’s isotopes to the rescue!
FAQs
How are elements structured?
Elements are like a big box of Legos, just waiting to be pieced together. Each element is made up of tiny units called atoms, which are composed of even tinier particles like protons, neutrons, and electrons. These atoms come together to form different structures like crystals, metals, or gases.
Why is understanding the structure of elements important?
Imagine trying to build a spaceship without knowing how to fit all the pieces together! Understanding the structure of elements helps scientists predict how different materials will behave in different conditions, leading to groundbreaking discoveries and inventions.
How do scientists explore the structure of elements?
Scientists are like detectives, using fancy tools like microscopes, spectroscopes, and particle accelerators to study the hidden secrets of elements. By bombarding them with energy or dissecting them atom by atom, scientists can unlock the mysteries of their structures.
What are some cool examples of element structures?
Ever heard of diamond? Its super-tight structure of carbon atoms makes it the hardest natural material on Earth! Or what about graphene? This one-atom-thick layer of carbon could revolutionize everything from electronics to solar panels. Element structures aren’t just science – they’re the building blocks of our world!
—
Let’s Dive Deeper!
Now that we’ve scratched the surface of the mysterious world of elements and their structures, it’s time to dive deeper and uncover even more secrets. Who knows what fascinating discoveries lie ahead? So grab your lab coat and safety goggles, because the adventure is just beginning! Stay curious, stay inquisitive, and keep exploring the amazing structure of the elements. Who knows what kind of scientific mischief we’ll get up to next? Happy exploring!