Welcome to the wacky world of pH, where acids and bases dance a delicate tango on a scale that can make your head spin faster than a chemistry professor on espresso. From lemon juice to bleach, understanding the pH scale is like deciphering a secret code to unlock the mysteries of everyday substances. So grab your goggles and hop on the proton train as we explore the highs and lows of balancing acids and bases like a boss. Let’s dive in and discover why pH is not just a trendy skincare ingredient, but a fundamental concept that keeps our world in tip-top shape.
The Importance of pH in Chemistry
pH in chemistry is like your ex in a bad breakup – you never know when it’s going to pop up and ruin your day. But unlike your ex, pH actually serves a purpose (shocking, I know). So, let’s dive into why pH is the unsung hero of the chemistry world.
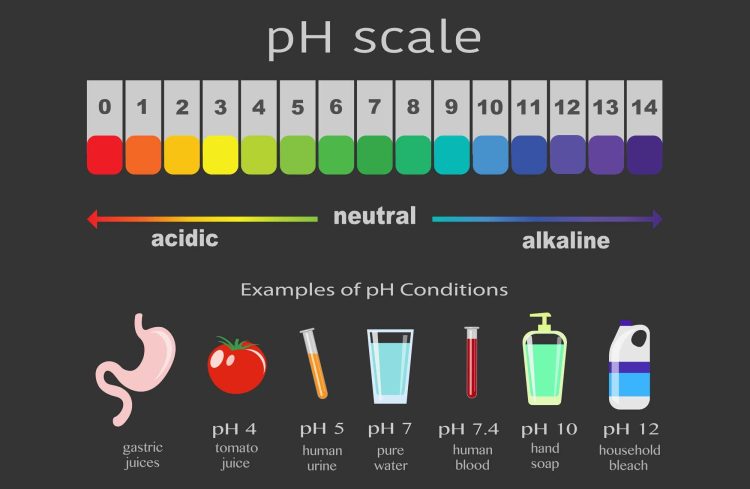
First things first, what even is pH? Well, it’s basically a fancy way of measuring how acidic or basic a solution is. pH stands for ”power of hydrogen”, because apparently, chemistry nerds couldn’t resist throwing some Greek letters into the mix. It’s measured on a scale from 0 (acidic as a lemon) to 14 (basic as your Aunt Mildred’s baking soda). Neutral solutions, like water, sit right smack dab in the middle at pH 7. It’s like the Goldilocks of the chemistry world – not too hot, not too cold, just right.
So why is pH so dang important? Well, for starters, it can tell you a lot about a substance without you even having to taste it (trust me, not a fun experiment). pH affects everything from how well your shampoo lathers to the color of your morning OJ. Plus, it plays a crucial role in biological processes, like making sure your blood doesn’t turn into a pool of acid (not a good look, just ask the Terminator).
Think of pH like the backstage crew of a Broadway show - without it, the whole production would fall apart. So, next time you see those two little letters, remember that pH may not be as flashy as a Hollywood star, but it’s definitely a chemistry superstar in its own right. pH, we salute you.
 Acids and Bases”>
Acids and Bases”>
Defining Acids and Bases
So, you might be wondering what exactly acids and bases are. Well, buckle up because we’re about to take a dive into the exciting world of chemistry!
First off, let’s talk about acids. Imagine acids as the villain in a superhero movie - they’re the ones causing all the chaos and destruction. Acids are substances that can donate a proton or accept an electron pair in a chemical reaction. They have a pH level of less than 7 and are known for their sour taste and ability to sting like a bee.
Now, let’s move on to bases. Bases are like the sidekick in the superhero movie – they’re there to save the day and neutralize the acidic villain. Bases are substances that can accept a proton or donate an electron pair in a chemical reaction. They have a pH level greater than 7 and are known for their bitter taste and slippery feel.
So, to sum it all up, acids and bases are like the Batman and Robin of the chemical world – opposites that work together to maintain balance. Whether it’s causing chaos or saving the day, acids and bases are essential players in the never-ending chemistry game.

Understanding the pH Scale
Ever wondered why lemons are so sour or why your stomach feels acidic when you eat too much junk food? Well, the pH scale is here to provide some answers! This magical scale measures the acidity or basicity of a substance on a scale from 0 to 14. The lower the number, the more acidic the substance, and the higher the number, the more basic it is. So basically, pH is like the mood ring of the chemistry world!
Now, before you start chugging baking soda to balance out last night’s spicy taco binge, let’s break down some pH basics. Here are a few key points to keep in mind:
- Neutral is a thing: A pH of 7 is considered neutral, just like Switzerland in the world of acidity. Pure water falls in this category, so don’t go accusing your H2O of being too basic!
- Acids are low-key divas: Anything with a pH lower than 7 is considered acidic. Lemons, vinegar, and your ex’s attitude all fall into this category. Caution: too much acidity may cause some serious burn, both literally and metaphorically!
- Bases are the chill ones: On the flip side, substances with a pH greater than 7 are considered basic. Think baking soda, soap, and your therapist’s calming voice during a meltdown. Bases are like the cool kid at the party who helps balance out the drama.
So there you have it, a crash course on the pH scale that even your high school chemistry teacher would appreciate. Just remember, when life throws you lemons (or any other acidic substance), make sure to neutralize the situation with some good ol’ basic vibes. Balance is key, my friends! Now go forth and conquer the acidic and basic battles of everyday life with the power of pH knowledge!

Measuring pH Levels
When it comes to , it’s important to remember that accuracy is key – unless you enjoy conducting experiments that end in explosions and chaos (in which case, carry on).
First things first, you’ll need a trusty pH meter. Think of it as your sidekick in the world of acidity and alkalinity – like Batman to your Robin, or salt to your margarita.
Next, make sure you calibrate your pH meter properly, because nobody wants a wonky reading that leads to questionable decision-making. And remember, just like relationships, pH levels can be a bit finicky – so be gentle and patient.
Once you’re all set up and ready to roll, dip that pH meter into your liquid sample like you’re conducting a top-secret spy mission (minus the turtlenecks and cool gadgets). And voila! Your pH level reading is revealed, guiding you through the mysterious world of chemical balance. Cheers to science!

Achieving pH Balance
Do you ever feel like your body is a rollercoaster of acidity and alkalinity? One minute you’re feeling as sour as a lemon, the next you’re as sweet as a peach. Well, it’s time to hop off that crazy ride and achieve some pH balance!
First off, let’s talk about the importance of pH balance. Our bodies function best when our pH levels are in harmony. When we’re too acidic, we can feel sluggish, bloated, and just overall blah. But when we’re too alkaline, we might be prone to infections and inflammation. It’s all about finding that Goldilocks zone.
So how can you achieve pH balance? Well, it’s not as complicated as you might think. Start by incorporating more alkaline foods into your diet, such as leafy greens, nuts, and seeds. And don’t forget to hydrate, hydrate, hydrate! Water is like the ultimate pH balancer.
And let’s not forget about the power of probiotics. These little guys work wonders for balancing out your gut flora and promoting a healthy pH level. So go ahead, indulge in some yogurt, kefir, or sauerkraut. Your body will thank you!
Effects of Imbalanced Acidity
Ever felt like your body is a giant science experiment gone wrong? Well, that might just be the case if you’re experiencing imbalanced acidity. Here are some of the wild effects it can have on your body:
- **Upset Stomach**: Your stomach might feel like it’s doing flips and cartwheels, thanks to the excess acid sloshing around in there.
- **Heartburn**: Ah, the classic burning sensation in your chest that feels like a tiny dragon is roasting marshmallows in your esophagus. Thanks, acidity!
- **Skin Woes**: Say hello to acne, redness, and irritation. Your skin might rebel against you if the pH levels are out of whack.
But wait, there’s more! imbalanced acidity could also lead to:
- **Bad Breath**: No amount of mints can mask the stench of acidity breath. Sorry, not sorry.
- **Fatigue**: Feeling tired all the time? Blame it on the acid-induced exhaustion.
So, if you’re feeling a bit off and suspect imbalanced acidity might be the culprit, it’s time to get that pH in check. Your body will thank you, and you can finally bid farewell to the acid-induced chaos!
Methods for Adjusting pH Levels
So you’ve discovered that your pH levels are a bit wonky and now you’re scrambling to figure out how to fix it. Fear not, dear reader, for I have some quirky methods up my sleeve that will have your pH levels back in line in no time!
First up, let’s chat about using good old baking soda. This magical white powder can work wonders when it comes to adjusting pH levels. All you have to do is sprinkle a bit into your water and watch as the pH levels magically balance out. It’s like a potion straight out of a wizard’s lab – but without the magical incantations.
Next on the list is everyone’s favorite citrus fruit – lemons! Squeeze some lemon juice into your solution and watch as the pH levels start to shift. Lemons are like tiny acidic superheroes, swooping in to save the day and bring balance back to your water. Who knew that a little lemon juice could be so powerful?
And last but not least, we can’t forget about our trusty friend vinegar. A splash of vinegar in your solution can work wonders when it comes to adjusting pH levels. It may not smell the greatest, but hey, beauty is pain, right? Just hold your nose and pour that vinegar in like the fearless pH warrior that you are!
FAQs
Why is understanding the pH scale important?
Well, if you ever want to stop accidentally creating explosive chemical reactions in your kitchen, then understanding the pH scale is kind of a big deal. Knowing how acids and bases interact can prevent disaster and help you create some pretty cool science experiments.
What exactly is the pH scale?
Think of the pH scale as a way to measure how basic or acidic a substance is. It ranges from 0 to 14, with 0 being super acidic (like your ex after a breakup) and 14 being super basic (like that one friend who is always way too happy).
How can I balance acids and bases in my everyday life?
If you’re tired of accidentally setting things on fire in your quest to bake a simple cake, then understanding the pH scale can help. By knowing how to balance acids and bases, you can avoid disaster and actually make something edible for once.
Can balancing acids and bases have practical applications beyond cooking?
Absolutely! From cleaning products to skincare, understanding the pH scale can come in handy in a variety of situations. Plus, it’s a great party trick to impress your friends with your newfound chemistry knowledge.
—
It’s All About That pH Balance!
Congratulations, you’ve made it to the end of our crash course on the pH scale! You’re now equipped with the knowledge to navigate the acidic and basic waters of chemistry like a pro. Just remember, when life gives you lemons, don’t forget to check their pH level first!
We hope you enjoyed this enlightening journey into the world of acids and bases. Now go forth and conquer those chemical reactions with confidence and a touch of pH sophistication. Stay balanced, my friends!