Welcome to the wacky world of heat exchange in chemical reactions! Have you ever wondered why some reactions get hot and bothered while others stay cool as a cucumber? Well, get ready to take a deep dive into the steamy science of how heat is exchanged during chemical reactions. So sit back, relax, and let’s turn up the heat on this sizzling topic!
Overview of Heat Exchange in Chemical Reactions
Heat exchange in chemical reactions is like a spicy salsa dance between molecules. Picture this: one molecule brings the heat, while the other molecule takes it in like a cool cucumber. It’s a hot and cold tango that keeps the reaction moving and grooving.
When molecules collide in a chemical reaction, they swap more than just witty banter. They exchange heat energy faster than you can say “exothermic reaction”! Some molecules are heat-hogs, soaking up energy faster than a sponge in a rainstorm, while others are heat-phobes, running away from it like a cat from a bath.
Think of heat exchange in chemical reactions as a game of hot potato, where molecules pass the energy back and forth like it’s a sizzling spud. Some molecules are happy to hold onto the heat, while others can’t wait to pass it on like a scorching relay race.
In the wild world of chemical reactions, heat exchange is the ultimate cha-cha-cha. It’s a dance of give and take, of hot and cold, of energy in motion. So next time you see a chemical reaction heating up, remember: it’s all about the heat exchange, baby.

Types of Heat Exchange: Endothermic and Exothermic Reactions
So, let’s talk about heat exchange, shall we? Now, when it comes to reactions, there are two types that you need to know about: endothermic and exothermic reactions. Sounds fancy, right? But don’t worry, I’ll break it down for you in a way that even your grandma could understand.
First up, we have endothermic reactions. These are like the energy vampires of the chemical world – they suck up heat like it’s going out of style. Picture a cold winter day when you’re huddled up in a blanket, sipping on hot cocoa. That’s basically what endothermic reactions are doing – absorbing heat from their surroundings to power themselves up.
On the other hand, we have exothermic reactions. These bad boys are the life of the party – they’re throwing heat around like it’s confetti. Imagine you’re at a BBQ and the grill is sizzling away, giving off that glorious warmth. That’s exothermic reactions in a nutshell – they release heat as a byproduct of their chemical shenanigans.
So, next time you’re cooking up a storm in the kitchen or mixing up some potions in the lab, remember the difference between endothermic and exothermic reactions. And who knows, maybe you’ll impress your friends with your newfound chemistry knowledge. Just don’t blame me if they start calling you a science nerd!

Factors Influencing Heat Exchange in Chemical Reactions
When it comes to heat exchange in chemical reactions, there are several factors at play that can impact the overall outcome.
One major factor is the type of reactants involved. Some reactants are like two magnets of the same pole – they repel each other and heat is released in the process. Others are like a match to a flame – they attract each other and heat is absorbed. It’s like a chemical romance, but with a lot more heat.
Temperature also plays a key role in heat exchange. Just like how people behave differently in hot and cold weather, reactants also react differently depending on the temperature. Sometimes they get all hot-headed and react violently, while other times they’re as cool as a cucumber.
Pressure is another factor that can influence heat exchange. It’s like squeezing toothpaste out of a tube – the higher the pressure, the faster the reaction goes. But be careful, too much pressure and things might just explode!

Measuring Heat Exchange: Calorimetry and Enthalpy
Calorimetry is the method scientists use to measure heat exchange during chemical reactions. It involves some fancy equipment and a touch of magic (just kidding, it’s all science!). By monitoring changes in temperature, we can determine the amount of heat transferred between substances. It’s like a high-stakes game of hot potato, but with molecules instead!
Enthalpy, on the other hand, is like the cool cousin of heat exchange. It’s a fancy term for the heat content of a system, taking into account pressure and volume. Think of it as the VIP section of the heat exchange club. Enthalpy changes during reactions can tell us a lot about the overall energy balance, kind of like a thermodynamic mood ring.
When you combine calorimetry and enthalpy, you get a powerful duo capable of unraveling the mysteries of heat exchange. It’s like Sherlock Holmes and Dr. Watson, but with test tubes and Bunsen burners. With their powers combined, they can crack the code on how heat moves through systems and uncover the hidden truths of chemical reactions.
So next time you find yourself pondering the mysteries of heat exchange, remember the dynamic duo of calorimetry and enthalpy. They may not wear capes, but they sure know how to heat things up in the world of chemistry!
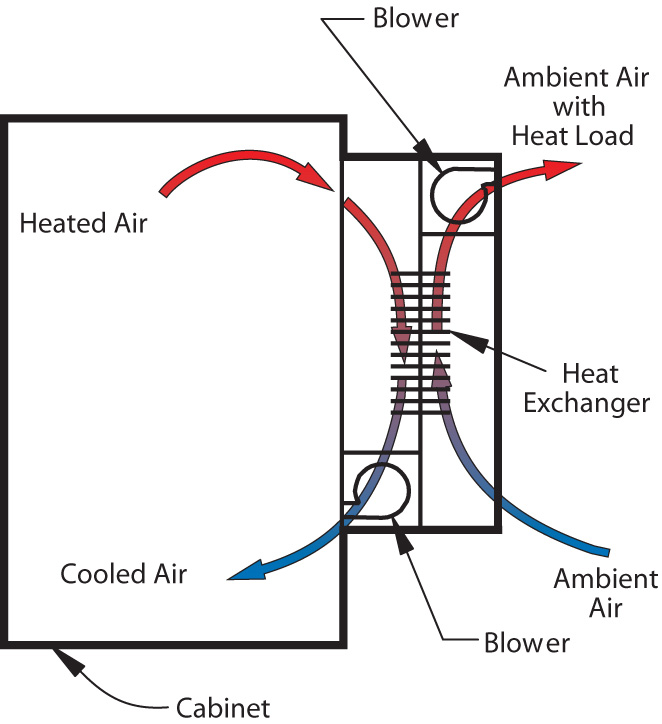
Uses of Heat Exchange in Industrial Processes
Heat exchange in industrial processes is like the unsung hero of manufacturing - quietly working behind the scenes to keep things running smoothly. Without heat exchange, our factories would be as dysfunctional as a toaster without electricity.
One of the most common is in cooling systems. From keeping machines from overheating to chilling out hot-headed employees, heat exchange is essential for maintaining a cool atmosphere. It’s like having a built-in air conditioner for your factory, minus the high energy bills.
Another handy application of heat exchange is in chemical processing. Imagine trying to mix chemicals without the precise temperature control that heat exchange provides. It would be like trying to bake a soufflé in a microwave – disaster waiting to happen. With heat exchange, you can keep those volatile reactions in check and avoid any explosive situations.
And let’s not forget about the efficiency benefits of heat exchange in industrial processes. By recycling heat and reducing energy consumption, heat exchange helps companies save money and reduce their carbon footprint. It’s like having a superhero on your team, fighting climate change one heat exchanger at a time.
Mitigating Heat Loss in Chemical Reactions
When it comes to chemical reactions, heat loss can be a real buzzkill. Not only does it slow down the reaction, but it can also lead to some pretty disastrous results if left unchecked. Lucky for you, there are a few tricks up our sleeves to help mitigate this pesky heat loss.
First things first, make sure to insulate your reaction vessel. This might sound like a no-brainer, but you’d be surprised how many chemists forget this simple step. By insulating your vessel, you can keep the heat where it belongs – in the reaction mixture. Plus, who doesn’t love a cozy reaction vessel?
Next, consider using a heat source like a hot plate or heat lamp to keep things toasty. Just be sure not to crank up the heat too high – nobody wants a reaction that’s hotter than the sun! Finding that sweet spot temperature can be a game-changer in mitigating heat loss.
Lastly, don’t forget to stir the pot – literally. By agitating your reaction mixture, you can help distribute heat more evenly throughout the solution. Plus, it’ll give you a killer arm workout. Two birds, one stone, am I right?
FAQs
Why is understanding heat exchange in chemical reactions important?
Because otherwise, your kitchen might explode while you’re trying to cook dinner…just kidding (hopefully). It’s actually crucial for understanding the efficiency and safety of chemical processes.
How does heat exchange affect chemical reactions?
Well, imagine trying to bake a cake without preheating the oven. You need the right amount of heat for things to actually happen. Heat exchange can speed up or slow down reactions, affect the equilibrium, and even determine the products formed.
What are some common ways heat exchange occurs in chemical reactions?
Think of it like a steamy love affair between reactants and products. Heat can be released (exothermic reactions) or absorbed (endothermic reactions) during a reaction. There’s also heat transfer through conduction, convection, and radiation.
How can one calculate heat exchange in a chemical reaction?
Get ready to flex those math muscles! You can use equations like q = mcΔT (for specific heat capacity) or ΔH = q / n (for enthalpy change). Just make sure your calculator is fully charged because things might get steamy.
What are some real-world applications of understanding heat exchange in chemical reactions?
Well, for starters, it’s essential in designing reactors for industrial processes like oil refining or pharmaceutical production. It can also help create more efficient energy storage systems or even spice up your next baking adventure.
—
In Conclusion: Saying Goodbye to the Heat Exchange Dance
And there you have it, folks! Understanding heat exchange in chemical reactions is like unraveling a thrilling mystery novel - except instead of detectives and villains, we have molecules and energy changes. Remember, when it comes to heat exchange, don’t be left out in the cold - stay hot on the trail of those exothermic and endothermic reactions. So until next time, keep those beakers bubbling and those bunsen burners burning!