Have you ever wondered what makes polar and nonpolar covalent bonds different, besides the fact that one is icy cold and the other just can’t make up its mind? Well, get ready to dive into the atomic world of connections as we compare these two electrifyingly different types of bonds. So grab your goggles and strap on your lab coat, because we are about to embark on a chemical journey that will leave you positively charged! Chemistry“>
Chemistry“>
Understanding Covalent Bonds in Chemistry
Covalent bonds are like the ultimate friendship bracelet in the world of chemistry. Two atoms decide to share their electrons like besties sharing secrets at a sleepover. This bond forms when two non-metal atoms get together and decide they can achieve stability by sharing electrons. It’s like a chemistry love story, but without the dramatic breakup.
Think of covalent bonds like a game of tug-of-war between atoms. Each atom wants to have a full outer shell of electrons, so they agree to share and play nice. This sharing of electrons creates a strong bond between the atoms, like a forever pact to stick together through thick and thin (or in this case, through chemical reactions).
Unlike ionic bonds where one atom steals electrons from the other like a sneaky thief in the night, covalent bonds are all about teamwork and collaboration. The atoms decide to work together and share the electron wealth, creating a stable and harmonious relationship. It’s like a chemistry version of a buddy cop movie, where the atoms team up to take on the world of molecules.
So next time you see two atoms happily sharing electrons and forming a covalent bond, just remember, it’s like a chemistry handshake – a symbolic gesture of partnership and unity in the vast world of atomic interactions. It’s all about sharing, caring, and creating strong connections in the wacky world of chemistry.
Key Differences Between Polar and Nonpolar Covalent Bonds
So you think you know everything about covalent bonds, huh? Well, buckle up because we’re about to dive into the polar versus nonpolar debate!
Let’s start with polar covalent bonds. These bad boys are like the popular kids in high school – they’ve got all the attention and everyone wants to be around them. Why? Because they involve the unequal sharing of electrons between atoms. It’s like that one friend who always hogs the spotlight in group photos.
On the flip side, we have nonpolar covalent bonds. They’re the quiet, introverted types that prefer to keep to themselves. In nonpolar bonds, electrons are shared equally between atoms, creating a harmonious equilibrium. It’s like a peaceful meditation session, where everyone gets a fair share of the zen vibes.
In summary, polar covalent bonds are like a dramatic soap opera, with all the drama and excitement. Meanwhile, nonpolar covalent bonds are like a cozy night in with a good book – peaceful and serene. So, which team are you on? The drama queens or the chill vibes? Choose wisely!

electronegativity-and-its-role-in-bond-polarity”>Electronegativity and its Role in Bond Polarity
Have you ever wondered why some atoms are so attractive while others just can’t seem to get along? Well, it all comes down to electronegativity. This sneaky little property determines how eagerly an atom will snatch up those precious electrons in a chemical bond.
When it comes to bond polarity, electronegativity plays a starring role. If two atoms with different electronegativities come together to form a bond, the electrons will spend more time hanging out with the atom that’s a real electron hog. This creates a lopsided situation where one atom gets a bit of a negative charge (thanks, electronegativity!) while the other is left feeling a bit positive about the whole affair.
Think of it like a possessive partner in a relationship – one atom just can’t help but take all the attention (and electrons) for itself. In a bond with similar electronegativities, the electrons are more evenly shared, creating a happy little family where everyone gets along (well, most of the time).
So, the next time you see a chemical bond forming, remember to thank electronegativity for keeping the peace (or stirring up some drama). It just goes to show – in the wild world of chemistry, there’s never a dull moment when electronegativity is involved!

Examples of Polar and Nonpolar Molecules
When it comes to molecules, there are two main categories: polar and nonpolar. The difference between the two comes down to how the atoms within the molecule share electrons. Let’s take a closer look at some examples of each to help you differentiate between the two.
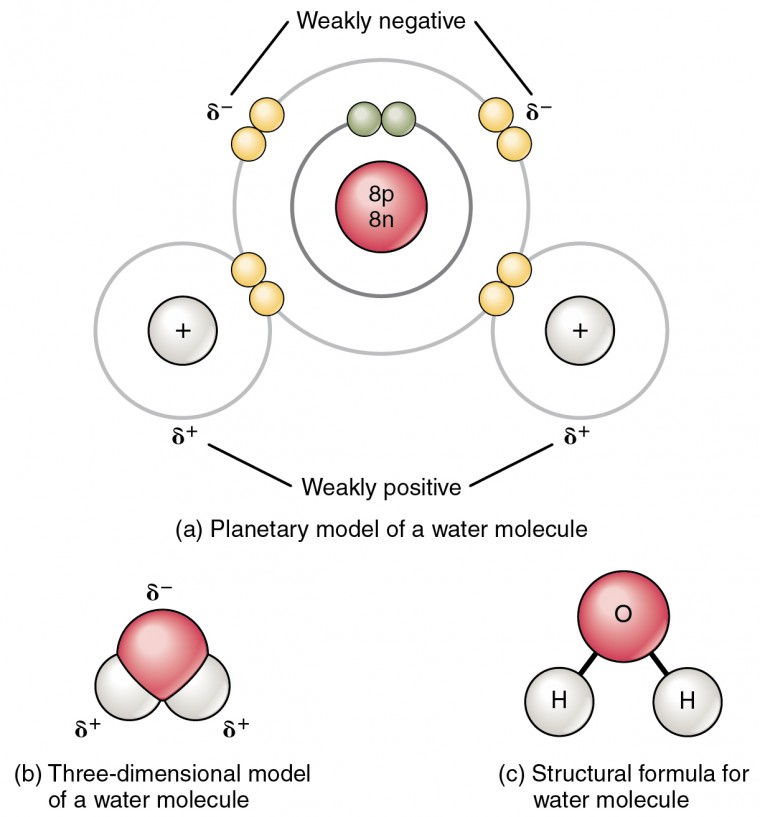
In the polar corner, we have water, the classic example of a polar molecule. Water is made up of two hydrogen atoms and one oxygen atom. The oxygen atom has a stronger pull on the shared electrons, causing the molecule to have a slight negative charge on the oxygen side and a slight positive charge on the hydrogen side. This makes water a polar molecule, which is why it is so good at dissolving other substances.
On the nonpolar side of things, we have methane. Methane is a simple molecule made up of one carbon atom and four hydrogen atoms. Because the atoms in methane share electrons equally, there is no separation of charge within the molecule, making it nonpolar. So next time you’re lighting a barbecue, remember that the gas fueling the flames is a prime example of a nonpolar molecule.
Another example of a polar molecule is ammonia. Ammonia is made up of one nitrogen atom and three hydrogen atoms. The lone pair of electrons on the nitrogen atom causes the molecule to have a slightly negative charge on the nitrogen side and a slightly positive charge on the hydrogen side. This polarity gives ammonia its distinct properties, like its strong smell and ability to act as a base.
The Influence of Molecular Shape on Bond Polarity
When it comes to bond polarity, molecular shape plays a crucial role in determining the distribution of electrons between atoms. Let’s dive into how the shape of a molecule can influence its bond polarity in a fun and quirky way!
First off, imagine a molecule as a group of friends going on a road trip. The more spread out and balanced the seating arrangement in the car, the happier everyone is! Similarly, when atoms in a molecule are arranged symmetrically, the electrons are shared evenly, resulting in a nonpolar covalent bond.
On the other hand, if the molecular shape resembles a lopsided game of Tetris, with one atom hogging all the electrons while the others pout in the corner, you’ve got yourself a polar covalent bond. This unequal sharing of electrons creates partial charges, giving the molecule a magnetic-like polarity.
So, remember folks, molecular shape isn’t just about looking pretty – it also determines whether a molecule is a party where everyone shares equally or a drama-filled reality show where one atom steals the spotlight. Stay tuned for more intriguing revelations about the wacky world of chemistry!
Comparing Physical Properties of Polar vs. Nonpolar Compounds
So you’re curious about the thrilling world of polar vs. nonpolar compounds, huh? Well buckle up, because we’re about to dive deep into the fascinating realm of physical properties!
First up, let’s talk about melting points. Polar compounds have higher melting points than their nonpolar counterparts. Why, you ask? Well, it’s all because of those pesky intermolecular forces. Polar compounds have stronger intermolecular forces (like hydrogen bonding) that hold them together, making it harder for them to break apart and melt. Nonpolar compounds, on the other hand, are more like a rowdy group of friends at a beach party – they can break apart and melt at lower temperatures because their intermolecular forces aren’t as strong.
Next on our list of comparisons is boiling points. Just like with melting points, polar compounds have higher boiling points than nonpolar compounds. This is because it takes more energy to overcome those strong intermolecular forces and turn a polar compound into a gas. So if you’re ever in a dilemma about whether to heat up a polar or nonpolar compound, just remember – the polar one will definitely take longer to reach boiling point!
And let’s not forget about solubility. Polar compounds are like social butterflies at a party – they love to mingle and dissolve in polar solvents like water. Nonpolar compounds, on the other hand, are like lone wolves at that same party – they feel more comfortable dissolving in nonpolar solvents. It’s all about finding your crowd, right?
FAQs
What’s the deal with polar and nonpolar covalent bonds?
Well, my friend, let me break it down for you. Polar covalent bonds occur when atoms share electrons unequally, creating partial positive and negative charges. Nonpolar covalent bonds, on the other hand, involve atoms sharing electrons equally, resulting in no charge separation.
How do you determine if a bond is polar or nonpolar?
Ah, the age-old question. To figure out the polarity of a bond, you need to look at the electronegativity of the atoms involved. If there’s a big difference in electronegativity, chances are it’s a polar bond. If they’re best buds with similar electronegativity values, it’s nonpolar.
What are some examples of polar and nonpolar covalent bonds?
Oh, I’m glad you asked! Water (H2O) is a classic example of a polar covalent bond because oxygen hogs those electrons like a squirrel hoarding nuts in winter. Carbon dioxide (CO2), on the other hand, is nonpolar because the carbons and oxygens play nice and share their electrons equally.
Do polar and nonpolar covalent bonds have any real-world applications?
You bet your buns they do! Polar covalent bonds are responsible for the unique properties of water, like surface tension and the fact that ice floats (mind-blowing, I know). Nonpolar covalent bonds are found in things like fats and oils, keeping our bodies well-lubricated and deliciously greasy.
—
In Conclusion: Where Do You Stand?
So, after learning about the electrifying world of polar and nonpolar covalent bonds, where do you stand? Are you team polar, with its unequal sharing of electrons causing drama and tension? Or are you team nonpolar, chillin’ with its harmonious and balanced electron sharing?
Whichever side you choose, just remember - when it comes to atomic connections, it’s all about finding that special bond. Thank you for joining us on this electrifying journey through the world of covalent bonds. Stay bonded, folks!






